Isotonic, Hypertonic, and Hypotonic Solutions
In this article, we will go over three types of solutions: isotonic, hypertonic and hypotonic solutions.
Before we go into the specific types, we will first go over the scenario in which the solution exists. For example, when we talk about the above solutions, these are solutions outside of a substance. For example, say if we place a cell in a solution, which is the example we will use for all the various solutions. The solution outside the cell is what we are referring to when we talk about isotonic, hypertonic, or hypotonic. The solution may be pure water or the solution may be water with a solute dissolved in it, or any such solution.
For the below examples, we will use a cell that has a NaCL concentration of 0.9%. So the water concentration inside
of it is 99.1%.
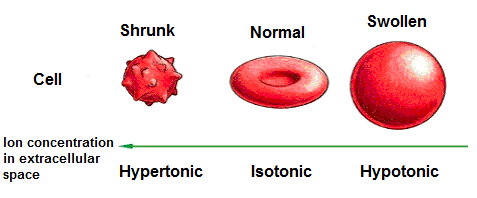
Isotonic Solution
An isotonic solution is a solution in which the same amount of solute and solution is available inside of the cell and outside
of the cell. The solution and solute percentage are the same inside the cell as it is in the solution outside
of the cell. Therefore, using the numbers above, a cell placed in a solution of water with 0.9% NaCL is in equilibrium.
Thus, the cell remains the same size. The solution is isotonic in relation to the cell.
Hypertonic Solution
A hypertonic solution is a solution that contains more solute than the cell which is placed in it. If a cell with a NaCl concentration of 0.9% is placed in a solution of water with a 10% concentration of NaCl, the solution is said to be hypertonic. Hyper means more, meaning that the solution that the cell is placed in contains more solute than the solution inside of the cell. When the solution contains more solute, this means that it contains less water. The solution outside of the cell is 10% NaCl, which means that it is 90% water. The solution inside of the cell is 0.9% NaCl, which means it is 99.1% water. Remember, solution flows from a higher concentration of water to a lower concentration of water. This is to dilute areas of higher solute concentrations, so that equilibrium can be achieved. Being that the outside solution is 90% water while the inside contains 99.1% water, water flows from the inside of the cell to the outside solution to dilute the high areas of solute concentration. Therefore, the cell loses water and shrinks.
Again, when we reference a solution to say it is hyper and hypo, we are referencing the amount of solute present in the solution
in comparison to the solute inside of the cell which is
in the solution. If the solution outside the cell has more solute than the solution inside of the cell, the solution is hypertonic.
If the solution inside of the cell has more solute than the solution outside of the cell, the solution is hypotonic. If the solution
outside of the cell contains the same solute as the solution inside of the cell, the solution is isotonic.
Hypotonic Solution
A hypotonic solution is a solution that contains less solute than the cell which is placed in it. If a cell
with a NaCl concentration is placed in a solution of distilled water, which is pure water with no dissolved substances it,
the solution on the outside of the cell is 100% water and 0% NaCl. Inside of the cell, the solution is 99.1% water and 0.9% NaCL. Water,
again, goes from a higher concentration to a lower concentration. So water goes from the distilled water solution to the inside
of the cell. As a consequence, the cell swells up and possibly bursts. Thus, putting a cell with solute in a distilled
water solution will cause swelling and possible bursting of the cell.
The main way to remember all of this is that when we talk about the various solutions, we are talking
in reference to the outside solution, not the solution inside of the cell. Then, next, when we talk about isotonic, hypertonic
and hypotonic solutions, we can use the prefixes and suffixes to determine which is which. The suffix -tonic is in relation
to the amount of solute in the solution. Hyper means more, hypo means below. So a hypertonic solution is a solution which contains
more solute than the solution inside of the cell. And a hypotonic solution is a solution which contains less solute than
the solution inside of the cell. This is the best way to learn this.
